Drugs ALS TDI Has Validated, Part 1: Copper ATSM
At the ALS Therapy Development Institute (ALS TDI), we discover and invent new drugs that could improve the lives of people with ALS, commonly known as Lou Gehrig’s disease. But as a nonprofit biotech company dedicated to finding effective treatments for ALS, it’s far from the whole picture. Our goal is to make sure that effective treatments for ALS make it from the lab to the clinic – whether they originated from our research or someone else’s.
While our major focus is on the invention of completely new ALS treatments, we also allocate some time and resources toward investigating the validity of promising results found in experiments by other researchers and companies. We do this by trying to replicate the experiments that were conducted in these other labs and testing to see if our experiments produce the same results. In drug discovery, this process is known as “validation.”
ALS TDI has attempted to validate many drugs over the years. While we always hope for positive results, in the majority of cases, we find that we are unable to achieve the same results.
However, in 2017, after 15 years of rigorously testing many drugs to confirm outside reports of their efficacy, we finally found one that appeared to live up to its published results: Copper ATSM (CuATSM).
What is CuATSM
CuATSM is a small molecule therapy that serves as a “copper chaperone.” Copper is an essential element to the function of human cells, and there is evidence that a copper deficiency may be a contributing factor in at least some forms of ALS, as well as other neurodegenerative diseases. However, taking copper on its own can be very toxic to the human body. CuATSM solves this issue with a small synthetic molecule that is able to safely deliver copper to damaged cells without harming the rest of the body.
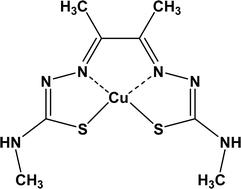
Initial Study of CuATSM
Researchers began investigating CuATSM, which had previously been used as an imaging agent, as a potential treatment for neurodegenerative diseases as far back as 2005. In 2016, two groups led by Dr. Joe Beckman at Oregon State University and Dr. Peter Crouch at the University of Melbourne published promising preclinical results from experiments in SOD1 mouse models of ALS. They found evidence that CuATSM could help prevent the misfolding of the SOD1 protein by delivering copper to safely to damaged cells, and appeared to be slowing the progression of the disease in the mice. Later that year, an Australian biotech company, Collaborative Medicine Development LLC, began a phase 1 clinical trial of the drug in Australia.
ALS TDI Study of CuATSM
After seeing the results from the University of Melbourne’s study on CuATSM, ALS TDI decided to investigate it further. Using our own SOD1 mouse models in our Cambridge lab, we recreated the conditions of the initial experiment. For 15 years, we had been conducting similar experiments investigating treatments developed by others, and every one had yielded results indicating that the treatment did not live up to the promise indicated in the initial findings. This time, however, was different: we found CuATSM really did appear to be slowing progression in the mice. The importance of this finding was summed up in the paper on the findings, published in 2017:
“Previously published reports describing the efficacy of a variety of potential therapeutics in SOD1 mice have not been repeatable in our labs using rigorously controlled testing methods, despite more than 15 years of testing,” wrote ALS TDI CEO and Chief Scientific Officer Fernando Viera and his coauthors. “Thus, CuATSM treatment is an interesting and important exception.”
According to Dr. Crouch, who conducted the initial experiment at the University of Melbourne, the validation by ALS TDI helped to raise excitement and interest in CuATSM as a potential ALS treatment.
“That was at the stage when we'd already published quite a few papers on the topic,” he says. “But I think it's fair to say that they weren't really registering with a broader audience. So, when the ALS TDI paper came out, that's when people started paying more attention to this particular compound as a potential option.”
In ALS, a condition where the mechanisms of disease are still poorly understood and many different treatment strategies are currently under investigation, independent validation from a respected research organization, like ALS TDI, can be a particularly important milestone.
“Ultimately, what we want is a lot of people testing a lot of different strategies, ” says Dr. Crouch. “But [knowing] how we can identify which ones are the ones that we should get excited about is actually quite challenging. That's why the ALS TDI validation was so critical, because that is an objective measure that you can point to and say, regardless of what you think about the drug or its mechanism or whether or not it's pertinent to ALS, here is a pretty hard and fast measure of veracity of the results.”
Current Status of CuATSM
Phase 1 trials for CuATSM were completed in late 2018. A Phase 2/3 study began enrolling in Australia in 2019 and is currently underway. For more information about CuATSM and to stay up to date on the latest updates about the trial, click here.
To learn more about ALS research at ALS TDI, visit www.als.net/als-research.